Pynazolam
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| Chemical and physical data | |
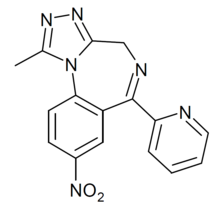
| Formula | C16H12N6O2 |
| Molar mass | 320.312 g·mol−1 |
| 3D model (JSmol) |
|
| |
InChI
| |
Pynazolam is a triazolobenzodiazepine derivative first invented in the 1970s,[1] which has in more recent years been sold online as a designer drug. Anecdotal reports and in silico studies suggest it has relatively potent hypnotic and sedative effects.[medical citation needed] Pynazolam is a powerful serotonin releaser similar to nimetazepam[2] which increases hypnotic activity and euphoria. Anecdotal evidence has also suggested that it's subjective effects are similar to methaqualone[3] although unlike methaqualone, it cannot be smoked due to high melting-point.
Limited pharmacological data is available[4] although their is an increasing availability of the compound for research purposes.[5]
See also
- Clonazolam
- Flunitrazolam
- Nitrazolam
- Pyeazolam
- Pyrazolam
- List of benzodiazepines
References
- ^ US 3970664, Sternbach LH, Walser A, "Preparation of triazolo benzodiazepines.", issued 20 July 1976, assigned to Hoffmann-LaRoche, Inc.
- ^ Pranzatelli, Michael R. (1989). "Benzodiazepine-induced shaking behavior in the rat: Structure-activity and relation to serotonin and benzodiazepine receptors". Experimental Neurology. 104 (3): 241–250. doi:10.1016/0014-4886(89)90036-8.
- ^ https://bluelight.org/xf/threads/flubromazolam-retrospective-6-years-with-the-knockout-night-nurse.892802/page-7
- ^ Catalani V, Floresta G, Botha M, Corkery JM, Guirguis A, Vento A, et al. (January 2023). "In silico studies on recreational drugs: 3D quantitative structure activity relationship prediction of classified and de novo designer benzodiazepines". Chemical Biology & Drug Design. 101 (1): 40–51. doi:10.1111/cbdd.14119. hdl:2299/25749. PMID 35838189.
- ^ https://pubchem.ncbi.nlm.nih.gov/compound/20368157
- v
- t
- e
- 2-Oxoquazepam
- 3-Hydroxyphenazepam
- Bromazepam
- BMS-906024*
- Camazepam
- Carburazepam
- Chlordiazepoxide
- Cinazepam
- Cinolazepam
- Clonazepam
- Cloniprazepam
- Clorazepate
- Cyprazepam
- Delorazepam
- Demoxepam
- Desmethylflunitrazepam
- Devazepide*
- Diazepam
- Diclazepam
- Difludiazepam
- Doxefazepam
- Elfazepam
- Ethyl carfluzepate
- Ethyl dirazepate
- Ethyl loflazepate
- Flubromazepam
- Fletazepam
- Fludiazepam
- Flunitrazepam
- Flurazepam
- Flutemazepam
- Flutoprazepam
- Fosazepam
- Gidazepam
- Halazepam
- Iclazepam
- Irazepine*
- Kenazepine
- Ketazolam
- Lorazepam
- Lormetazepam
- Lufuradom*
- Meclonazepam
- Medazepam
- Menitrazepam
- Metaclazepam
- Motrazepam
- N-Desalkylflurazepam
- Nifoxipam
- Nimetazepam
- Nitemazepam
- Nitrazepam
- Nitrazepate
- Nordazepam
- Nortetrazepam
- Oxazepam
- Phenazepam
- Pinazepam
- Pivoxazepam
- Prazepam
- Proflazepam
- Quazepam
- QH-II-66
- Reclazepam
- RO4491533*
- Ro05-4082
- Ro5-4864*
- Ro07-5220
- Ro07-9749
- Ro20-8065
- Ro20-8552
- SH-I-048A
- Sulazepam
- Temazepam
- Tetrazepam
- Tifluadom*
- Timelotem*
- Tolufazepam
- Triflunordazepam
- Tuclazepam
- Uldazepam
 | This sedative-related article is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e









